how to draw molecular orbital diagram of no
Since more than one atom is involved we refer to these orbitals as molecular orbitals. Molecular orbital theory diagram for.
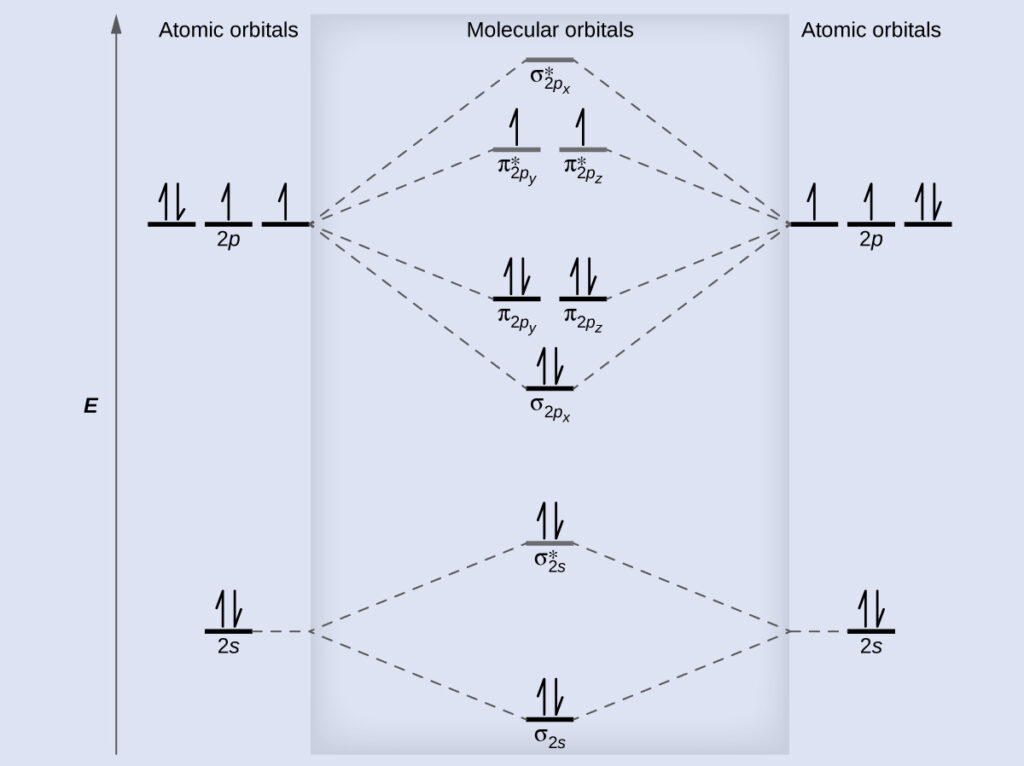
Molecular Orbital Diagrams Bond Order And Number Of Unpaired Electrons Chem Textbook
Mar 4 Find an answer to your question Draw and explain the molecular orbital diagram of Ne2.
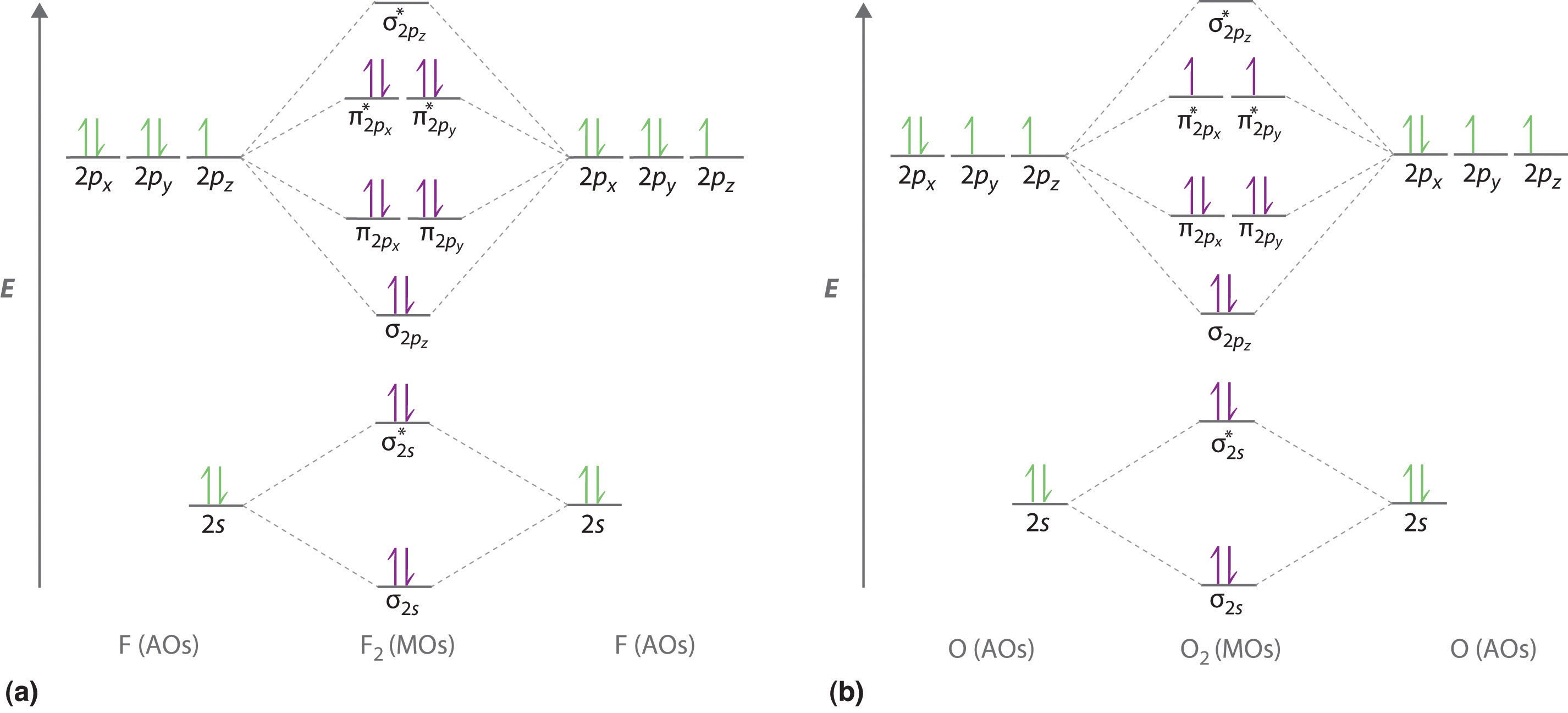
. 2 posts Page 1 of 1. Depending on if it is a homonuclear case where the bonding atoms are the same or a. Molecular orbital diagram of N 2 is shown below.
MAKE SURE TO SUBSCRIBEThis video puts emphasis on molecular orbital diagrams a fundamental way of understanding why Diels-Alder chemistry works. Since more than one atom is involved we refer to these orbitals as molecular orbitals. Return to Molecular Orbital Theory Bond Order Diamagnetism Paramagnetism.
The alignment of the atom. Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. Click bellow CHANNEL LINK to subscribehttps.
According to Molecular Orbital theory only those molecule can exists which have net positive bond order while the molecules with negative or. AO2px AO2px π2px π 2px weak sidelong overlap AO2py AO2py π2py π 2py weak sidelong overlap AO2pz AO2pz σ2pz σ 2pz strong head-on overlap Thus we take 10 atomic orbitals and generate 10 molecular orbitals in. Draw the complete molecular orbital diagram for eqC_2 eq form the molecular orbital diagram from the combination of a neutral C and a cationic eqC eq.
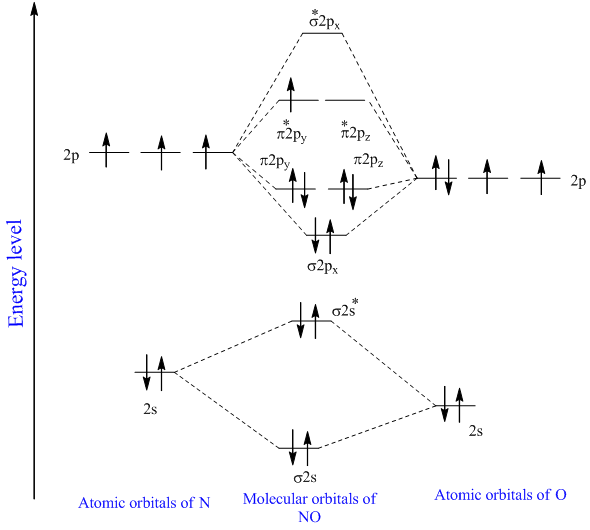
See Resources for a diagram showing the filling order. The last electron is in fact in the 2b_1 antibonding MO so the bond order of NO has decreased by 12 relative to NO or CO. 8 - Drawing Molecular Orbital Diagrams.
Draw and explain all the properties of CO and NO with the help of molecular orbital diagram. Abstract TLDR Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. Draw a circle around the nucleus.
Procedure to draw the molecular orbital diagram of CN. When considering bonding between two atoms first construct the relevant molecular orbitals then fill them with the available electrons starting with the lowest energy molecular orbital. 2 So the formula to find bond order is Bond order dfrac12 Number of electrons in BMO Number of electrons in ABMO Bond order dfrac12 8 2 Bond order dfrac12 6 Bond order 3 - N_2 molecules are diamagnetic with no unpaired electrons.
Molecular Orbital Diagram For Ne2. These can be further customized as you will learn in the next section. Procedure to draw the molecular orbital diagram of CN.
Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. The MO diagram of NO is. Clearly CN is hetero orbital.
Each orbital can accommodate a maximum of two electrons but. This command has two parameter in the example. Determine point group of molecule if linear use D2hand C2vinstead of Dhor Cv 2.
For more informative Chemistry Lessons Subscribe DIGITAL KEMISTRY. Draw the molecular orbital diagram for NO 3-Expert Solution. Draw a small circle and write the symbol in the centre.
Draw another circle around the first shell. N-O Hence the bond order of NO is either 25 or 35 depending on whether the last electron went into a bonding or antibonding MO. Add up to two electrons to the first electron shell.
Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. Find the valence electron of each atom in the CN molecule.
Check out a sample QA here. Note that the n 1 level only has s orbitals the n 2 level only has s and p orbitals and the n 3 level only has s p and d orbitals. Next well see that symmetry will help us treat larger.
Compare the bond order in h 2 and h 2 using the molecular orbital energy diagram for h 2. Find the characters of the reducible representationfor the combination of. 1s 2s 2p Are the energy sub-levels to be drawn.
Want to see the full answer. Bond order can be calculated by the formula. Open an example of the MOdiagram package in Overleaf.
If non-linear yaxes of outer atoms point to central atom 3. How to draw an electron configuration diagram Find the element on the periodic table. So no electrons should be in that orbital and then finally once you have everything drawn fill the molecular orbitals according to the rules of electron configuration which would be Aufbau principle you have to build up Pauli exclusion you can only put two electrons in each orbital and hunds rule you have to fill or equal energy orbitals.
When two or more atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. In general I dont think it matters where you take the electron from because it has no affect which side when drawing the molecular orbital diagram. Bond order bonding electrons - antibonding electrons 2.
On the basis of molecular orbital diagram explain. Number of electrons in antibonding orbitals. Assign x y zcoordinates zaxis is principal axis.
Orbitals represented by are antibonding orbitals and the orbitals without are bonding orbitals. 8 - Drawing Molecular Orbital Diagrams Flux Science. Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom.
The basic command to draw MO diagrams is atom. The Aufbau principle tells you that the lowest-energy orbitals fill first but the specific order isnt sequential in a way thats easy to memorize. Clearly carbon has 4 valence electrons and nitrogen has 5.
Add up to eight electrons to the second shell. To obtain the bond order look at the molecular orbitals formed and decide whether they are bonding or antibonding. This picture shows the molecular orbital diagram of N 2.
Solved Chapter 5 Problem 7p Solution Inorganic Chemistry 5th Edition Chegg Com

Draw The Molecular Orbital Diagram Of N2n2 N2 Write Class 11 Chemistry Cbse
Draw Mo Diagram Of Co And Calculate Its Bond Order Sarthaks Econnect Largest Online Education Community

Schematic Molecular Orbital Diagrams Of Al 2 And Alsi For Simplic Download Scientific Diagram
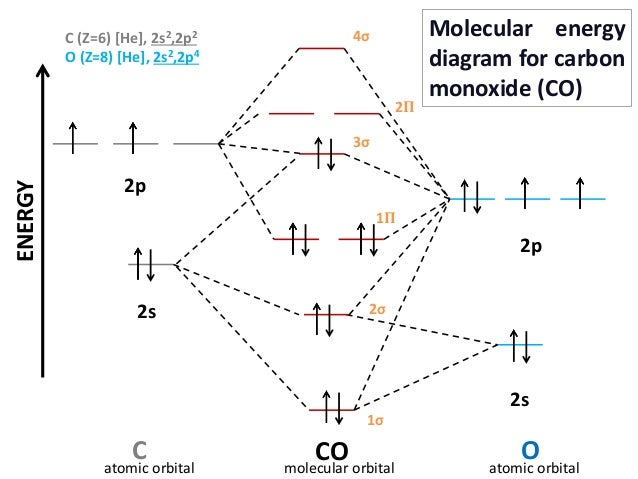
Molecular Orbital Diagram Of Co And No

Tikz Pgf Molecular Orbital Diagrams In Latex Tex Latex Stack Exchange

2 6 Molecular Orbital Theory Chemistry Libretexts
Draw The Diagrams For No 2 No 2 And No 2 The Homo Of No 2 Shows It Is Somewhat Anti Bonding Would You Expect The Nonbonding Electron Pairs On Nitrogen Or Oxygen To Be More Reactive
Solved The Nitrosyl Ion No Has An Interesting Chemistry Assume The Molecular Orbital Diagram

Mathematics Origins Of Molecular Orbital Diagrams History Of Science And Mathematics Stack Exchange
Explain The Mo Diagram For No Molecule Sarthaks Econnect Largest Online Education Community

Construct Molecular Orbital Diagram And Determine Unpaired Electrons In O2 O2 Bn No Study Com

Tikz Pgf Molecular Orbital Diagrams In Latex Tex Latex Stack Exchange

Delocalized Bonding And Molecular Orbitals

Draw The Molecular Orbital Diagram Of O2 And Calculate The Bond Order Is O2 Diamagnetic Or Paramagnetic Explain Your Answer Study Com
Molecular Orbitals Introductory Chemistry

Molecular Orbital Diagram For No Download Scientific Diagram
